IMPORTANT SAFETY INFORMATION
PANHEMATIN is contraindicated in patients with known hypersensitivity to this drug.
Risk of Phlebitis: Phlebitis is possible. Utilize a large arm vein or a central venous catheter for administration to minimize the risk of phlebitis.
Iron and Serum Ferritin: Elevated iron and serum ferritin may occur. Monitor iron and serum ferritin in patients receiving multiple administrations of PANHEMATIN.
Anticoagulant Effects: PANHEMATIN has transient and mild anticoagulant effect. Avoid concurrent anticoagulant therapy.
Renal Effects: Reversible renal shutdown has been observed with an excessive hematin dose (12.2 mg/kg in a single infusion). Strictly follow recommended dosage guidelines.
Transmissible Infectious Agents: PANHEMATIN may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. There is also the possibility that unknown infectious agents may be present in the product.
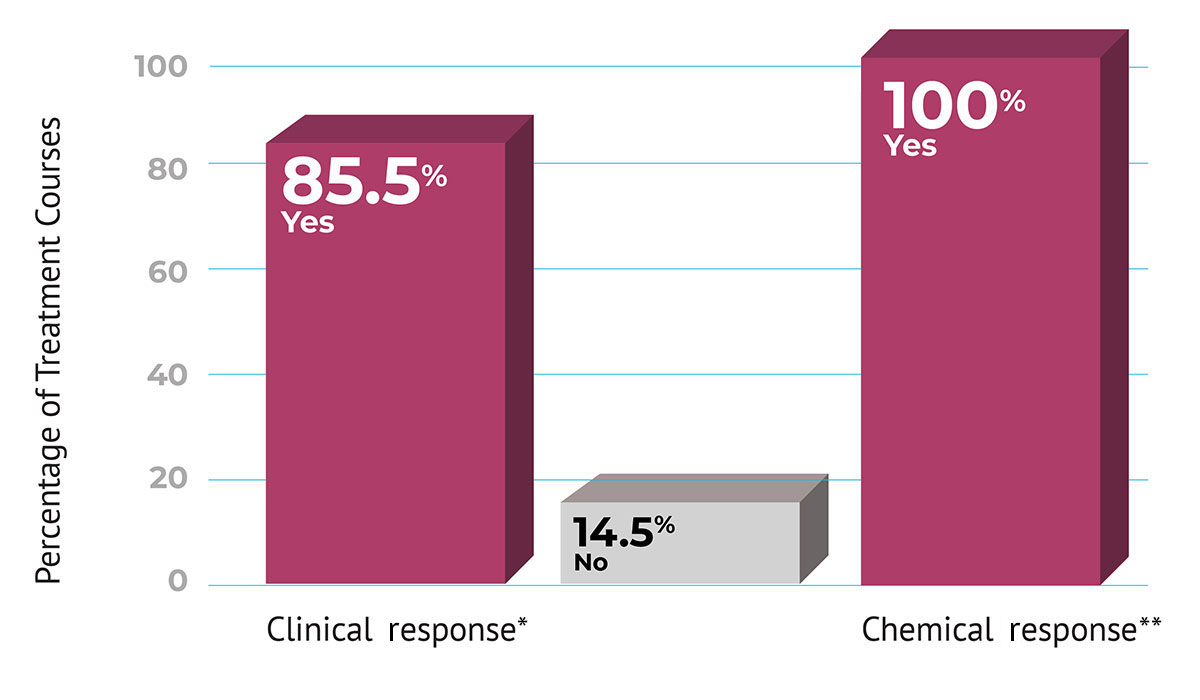
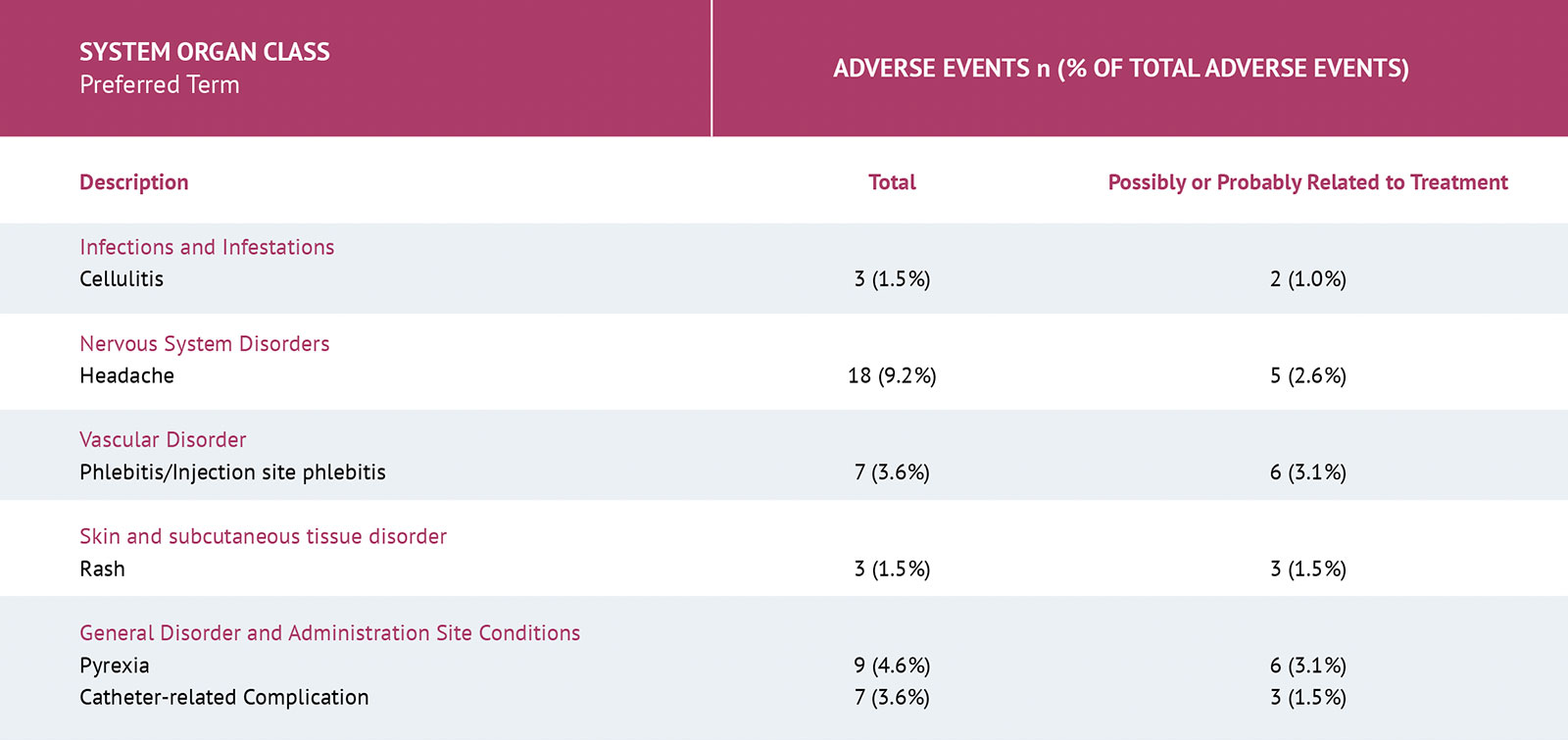
The most common adverse reactions (>1% of patients) are headache, pyrexia, infusion site reactions, and phlebitis.
To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions: Avoid CYP inducing drugs such as estrogens, barbituric acid derivatives and steroid metabolites which induce δ-aminolevulinic acid synthetase 1 (ALAS1) through a feedback mechanism.
PANHEMATIN® (hemin for injection), for intravenous infusion only, is available as powder for reconstitution in 350 mg vials.